|
Immune Surveillance
For
many years, scientists have
debated whether our immune
systems can identify cancer
or other abnormal cells and
destroy them spontaneously.
This concept, called “immune
surveillance,” suggests that
throughout life the immune
system constantly examines
the surfaces of cells in the
body and can tell when a
cell becomes abnormal. When
it finds an abnormal cell,
the immune system has
machinery that can attack
that cell and destroy it.
This clearly can happen with
cells infected by viruses,
or with cells and tissues
transplanted from a
non-matched individual.
However, there has been
debate as to whether this
happens constantly with
cancer cells. Further, there
has been debate as to
whether a failure of this
system actually could have a
role in leading to
clinically significant cases
of human cancer.
Many years ago, scientists
developed mice with defects
in their immune systems,
such as “nude mice.” If
immune surveillance were
important, one might expect
that mice with poor immunity
might have a higher
incidence of spontaneous
cancers. Surprisingly, when
these nude mice were
followed for a long time,
only a few rare tumor types
developed. This result
dampened enthusiasm for the
existence of such a
surveillance system.
However, in recent years,
other types of immune
deficient mice have been
developed and it is now
understood that the immune
system is more complicated
than first thought. The nude
mice, for example, while not
having T-cells, do have an
intact “innate” immune
system of other cell types,
and are not really
completely immunodeficient.
When completely immune
deficient mice were
examined, such as ones that
are missing the genes for
perforin as described
earlier, they do, in fact,
develop common tumors at a
faster rate. As a result,
the last decade has seen a
renewed interest in the
concept of immune
surveillance.
The concept suggests that as
cancer cells develop, they
are detected by the immune
system at a very early stage
(perhaps at the stage of a
single cell or a few cells)
and are then killed and
cleared from the body. This
actually makes some sense
from what we now know about
the properties of cancer
cells. A hallmark feature of
cancer cells is the loss of
control of the accurate
duplication of their genes,
such that they constantly
gain mutations. Those
mutations that are an
advantage to the cancer cell
will allow it to survive, so
cancers constantly become
more and more bizarre as
they develop. Such bizarre
changes should make the
surface of cancer cells very
different from normal cells
and make them easier for the
immune system to detect.
This assumes, however, that
the cancer cells also do not
develop some other property
that makes them either
invisible to the immune
system or actively able to
kill immune cells. Both of
these defensive tricks by
cancer cells may occur.
This concept implies that we
are constantly getting
cancer (one cell at a time)
but the cancer cells do not
survive because our immune
systems detect and kill
them. But only the cancer
cells die; normal cells are
unharmed. That this could
happen with such precision
was at first difficult to
believe. However, the SR/CR
mouse is a direct
demonstration that such
immune-mediated killing can
occur in an otherwise
healthy animal. For this
reason, this unique mouse
provides another bit of
evidence that immune
surveillance is probably a
normal process that protects
us from constantly
developing cancer.
Cancer Resistance Genes
This mouse also shows that,
just as immune deficiency
can be genetically
determined (inherited immune
deficiencies), immune
protection can also be
genetically determined. We
all know that the risk for
some types of cancer can be
inherited, such as families
who have a high frequency of
breast cancer, ovarian
cancer, etc. Most of the
identified familial risk
genes are ones that cause
tumor cells to grow and
survive. Few have been
identified that affect how
our immune systems can
influence cancer incidence
although, some inherited
immune deficiencies do
increase the risk of
developing certain forms of
cancer.
On the other hand, how would
we know that a particular
family has a gene that
resists cancer? Rather than
having a higher incidence of
cancer, that family would
simply not have cancer.
Could we spot such a family?
Probably not, since we think
of not having cancer as a
normal characteristic. The
only way to identify such
people might be to look at
someone with high risk for
cancer development, such as
through some form of risky
exposure (environmental
carcinogens) and/or old age
(where cancer statistically
would be more common).
The SR/CR mouse demonstrates
that cancer resistance genes
exist and can have dramatic
effects on responses to
cancer development. This
suggests that we should look
more carefully for such
cancer resistance genes in
people, since identifying
them (or their absence)
could tell us a great deal
about how susceptible to
cancer we really are.
Why Does Cancer Incidence
Rise with Increasing Age?
The difference in efficiency
of the cancer resistance
mechanism in the SR/CR mice
with aging brings up an
interesting concept. We
think of the increased
incidence of cancer in the
aging population as a
demonstration that mutations
are constantly occurring
during our lifetimes. It is
certainly true that many
cancers actually develop
over many years before they
become clinically
detectable. It is also true
that many cancers develop as
the result of accumulation
of stepwise multiple
mutations in important genes
that control growth of
cancer cells. But is this
the whole story?
The SR/CR mice show
resistance at an early age
to cancer cells that have
developed advanced and
bizarre mutations that allow
them to grow in normal mice.
Yet, the immune system in
the SR/CR mice still can
kill them all. But as the
SR/CR mice age without being
exposed to tumor cells, they
naturally lose the
effectiveness of this
resistance mechanism. Could
the same thing influence
cancer development in
people? Could it be that we
constantly reject cancer
cells by immune surveillance
throughout our lives and, as
we age, that mechanism
becomes weaker and weaker,
until finally one cancer
cell overcomes those
controls? The SR/CR mouse
provides support for the
concept that cancer develops
not only because of
accumulated mutations in
cancer cells, but also
because immune rejection
begins to fail with age. By
understanding how this mouse
rejects cancer when it is
young, we may be able to
boost the immune rejection
of cancer cells in later
life. This might make it
possible to do a similar
thing in people, and add to
our weapons to fight disease
in cancer patients.
Spontaneous Regression of
Human Cancer
For many years, reports of
the spontaneous
disappearance of advanced
cancer have appeared in the
scientific literature. While
some could be dismissed as
mistaken diagnoses, or
unexpected treatment
successes, there is a clear
body of evidence that such
things do, in fact, occur.
It happens so rarely that
scientists have no hope of
being able to study it,
since the patient is cured
at the time it is recognized
and there is no way to do
further investigation. What
has been lacking in this
field is an appropriate
animal model in which the
regression events could be
repeatedly produced and
studied in detail. The SR/CR
mouse is perhaps such a
model and demonstrates at
least one way in which this
event could happen. Further,
it is controlled genetically
in an otherwise healthy
animal.
Potential Therapies of Human
Cancer
The SR/CR mouse is only an
animal model. Its use is
limited to the laboratory
and perhaps only special
circumstances, such as
transplantation of mouse
cancers. For example, we
have yet to show that this
mouse could reject a tumor
that developed in its own
tissues, although those
experiments are currently
under way. Considering that
the transplanted tumors used
previously are from
essentially genetically
identical animals, it is
probably likely that
rejection of naturally
occurring tumors will occur.
However, a more compelling
question is how the
knowledge gained from this
mouse could be used to
benefit human patients.
There are several
possibilities. First, the
mutation in this mouse will
be identified in the future,
and a similar gene almost
certainly exists in humans.
Whether a similar mutation
introduced into that human
gene would have the same
benefit is unknown, but that
is the first possible
strategy to employ this
knowledge. For example, one
could take white blood cells
of the appropriate type from
a cancer patient and one
could introduce that mutated
human gene into the
patient’s cells outside of
the body (ex
vivo), and then
give them back to the same
patient. In this way,
perhaps, the transferred
immune cells might be more
effective against the
patient’s cancer. Such
strategies are complex and
will take many years to
develop.
Another strategy is to
understand the pathways and
mechanisms that are used in
the mouse to detect and
reject cancer cells, and
then employ those in a more
general way in patients.
That is, if a drug could be
developed that would enhance
a similar immune system
component in people, it
might be predicted to be an
effective anti-cancer
therapy.
Another concept is that the
loss of resistance to cancer
with aging in this mouse may
be under some form of
predictable regulation. The
mouse model may provide a
way of understanding how
aging affects immune system
function. With that
knowledge in hand, it might
be possible to prevent that
loss of function and
decrease the rate of
development of human cancer.
Conclusions
Whether this mouse model can
yield important clues to
human cancer will await
future experimental results.
There are some features,
however, that give reasons
for at least guarded
optimism. The resistance
trait is dominant (only one
copy of the mutation is
needed to see the effect),
is very dramatic, and is
effective against highly
aggressive forms of
experimental cancer. The
mice are otherwise healthy
and have a normal lifespan.
The mechanism responsible
for resistance can be
boosted and can keep older
animals resistant much
longer than they otherwise
would be. In studying this
mouse model, we start from a
highly successful
“phenotype” and only have to
work back to understand what
is already a successful
mechanism. Nature has done
the hard part in creating
the mutation; it is only up
to the scientists studying
the mouse to keep an open
mind and understand how it
did it. We can only be
grateful that Nature never
read our textbooks.
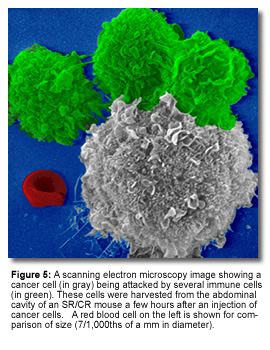 |

